THYMUSKIN®
An overview of key study findings
Studies and controlled observational scientific investigations performed on more than 1,000 men and women at dermatology and university clinics in Germany and internationally have verified the effectiveness of Thymuskin on all forms of hair loss. The success rate for androgenetic hair loss, telogen effluvium, alopecia areata and mild forms of cytostatic drug-induced alopecia averages well over 90%, with no side effects involved even with long-term use. The most important study findings have been compiled into this pamphlet.
 Thymuskin is a dermatological system to counter hair loss and to activate new hair growth for both men and women. It is highly effective, offers very good tolerability for various types of hair loss and does not have any side effects.
Thymuskin is a dermatological system to counter hair loss and to activate new hair growth for both men and women. It is highly effective, offers very good tolerability for various types of hair loss and does not have any side effects.
The dermatological Thymuskin system consists of the combined application of Thymuskin Shampoo and Thymuskin Scalp Serum. Thymuskin contains the patented GKL-02 active complex which mimics natural thymus extract and is free of animal-derived ingredients.
The GKL-02 thymic peptide active complex improves supply to hair follicle cells during the growth phase, boosting the formation of new hair cells and strengthens those already in place. It also extends the hair's growth phase. Hair follicles remaining intact are reactivated, new hairs are formed and the growth phase is significantly extended. In addition, Thymuskin boosts immune defenses and reduces the number of follicle cells dying off.
Thymuskin has been effective against hair loss for more than 30 years - a track record which is unique worldwide. This lasting success is based on the intensive research and study work undertaken by the Klett-Loch company group.
Overview of THYMUSKIN® Clinical Studies
- Lainz Hospital, Vienna
1st Department of Surgery
Ludwig Boltzmann Institute of Clinical Oncology Prof. Dr. H. Denck - Hannover Medical School
Department of Haematology/Oncology
Dr. med. H. Wilke - Women Dermatologists of Italy (DDI)
Part I (12 weeks), part II (24 weeks) Dott. Corinna Rigoni
- Lainz Hospital, Vienna
Women's Clinic, Department of Gynecological Endocrinology Prof. Dr. med. Dr. h.c. B. Runnebaum - University Hospital Muenster
Surgical Oncology
Prof. Dr. Dr. N.-P. Lüpke - Medical Clinic and Outpatient Clinic at TU Munich
'Klinikum rechts der Isar' University Hospital Department of Oncology/Haematology
Prof. Dr. med. Fink
- Institute of Pathophysiology and Transplantation
University of Milan, Italy
Dott. Mauro Barbareschi - German Cancer Research Centre
Department of Medical and Biological Informatics Prof. Dr. Claus O. Köhler - Darmstadt Clinic - academic teaching hospital
for Frankfurt/Main and Heidelberg-Mannheim Universities Director of the Dermatology Clinic
Prof. Dr. med. Hagedorn
The THYMUSKIN® Active Complex
In addition to natural substances and cosmetic ingredients, Thymuskin also contains the patented active thymic peptide complex GKL-02.
Unique worldwide, this active complex is the result of many years of research. In this peptide library thirty three amino acid derivatives are chemically combined in a statistic manner with each other and lastly physically combined with seventeen naturally occurring amino acids, either as such, as their salts and as their derivatives. This results in a unique active substance that contains di-, tri-, tetrapeptides as well as free amino acids. The peptides in the GKL-02 active thymic peptide complex were created using a peptide synthesis process (rather than being extracted from animal tissue) and therefore contain no animal components. The composition of this statistical peptide library mimics that of calf thymus gland total extract.
The average molecular weight of the individual peptides is between 180 and 600 daltons, thus facilitating penetration through the follicle skin layer after being applied to the scalp. As the GKL-02 active thymic peptide complex contains numerous components which act synergistically, the mode of action cannot be attributed to a single peptide; instead, the active complex as a whole is responsible for the various biological effects on hair growth. These effects have been successfully proven in numerous preclinical and clinical studies.
THYMUSKIN® Mode of Action
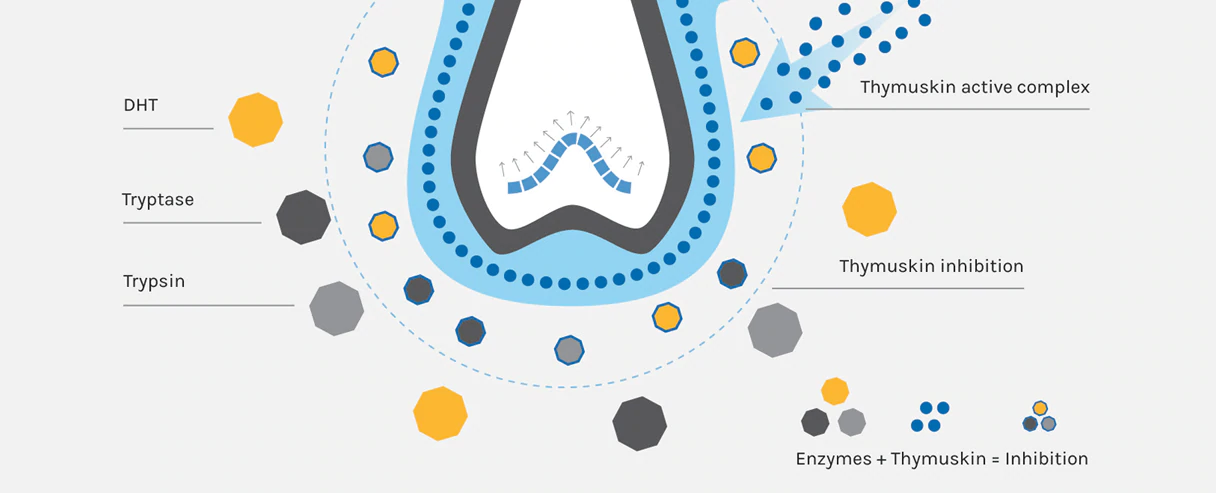
Thymuskin has an immunological effect on hair follicles, as is typical for a thymic preparation. The innovative peptide synthesis technology enables the peptides to penetrate deeply into the skin and to reach the hair follicle without any difficulty.
Thymuskin stops hair loss and stimulates new hair growth by increasing keratinocyte cell viability, boosting lymphocyte proliferation, inhibiting the effect of mast cells on hair follicles, and directly modulating hair follicle growth.
Preclinical Studies
Studies performed on human blood cells have shown that Thymuskin stimulates lymphocytes to divide. This effect was dependent on the concentration administered, and was statistically significant compared with the untreated group (p=0.03). The mechanism behind this stimulation is unrelated to interleukins.1 The increased proliferation of lymphocytes is most likely connected to immunological regulation and modulation of hair growth. Autoimmune processes play a particular role in alopecia areata.
Clinical Studies
A multicentric study8 was undertaken investigating the effectiveness and tolerability of Thymuskin (serum and shampoo) in the initial phase of androgenetic alopecia (AGA) and chronic telogen effluvium (chronic TE) over a period of six months. The study included 364 test subjects split as follows:
- 70 men (average age 30.7) with AGA
- 57 men (average age 35.9) with chronic TE
- 53 women (average age 42.1) with AGA
- 184 women (average age 36.9) with chronic TE
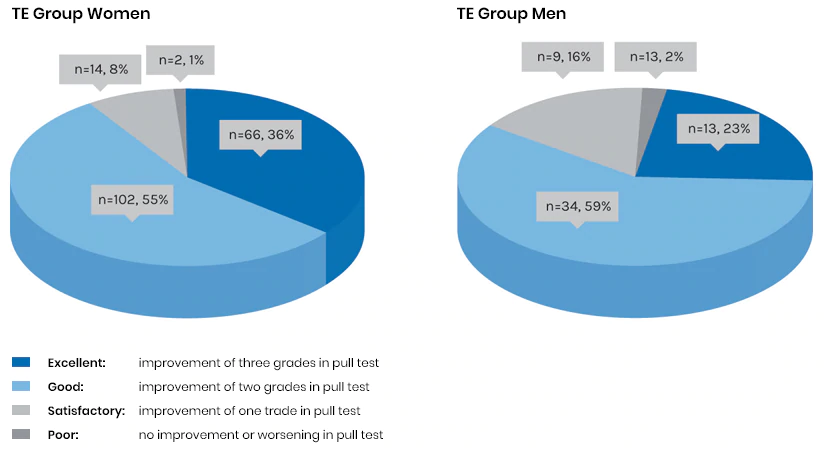
The patients used the serum once a day and the shampoo three times a week over a total period of 24 weeks. All test subjects underwent a pull test and a symptom evaluation (seborrhoea, erythema and itching) performed by the investigator. Tolerability and cosmetic acceptance/tolerance were also assessed. After six months of treatment, patients with AGA and patients with chronic TE both demonstrated a significant decrease in hair loss, a stimulation of hair growth and a reduction in the symptoms of seborrhoea, erythema and itching. Tolerability and cosmetic acceptance were good to very good in the majority of patients.
1. Reduction in hair loss

Fig. 4: Hair loss assessment in pull test8
2. Decrease in seborrhoea and erythema
- Seborrhoea and erythema improvement in 93% and 97% of women respectively
- Seborrhoea and erythema improvement in 89% and 90% of men respectively

Fig. 5: Seborrhoea and erythema symptom improvement
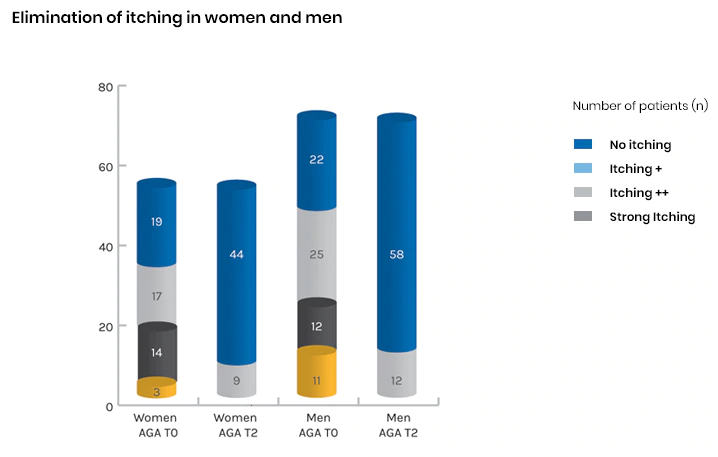
3. Elimination of itching
- Itching eliminated in 74% of women (25/34), with the remaining 26% (9/34) still experiencing light itching
- Itching eliminated in 75% of men (36/48), with the remaining 25% (12/48) still experiencing light itching
- Strong itching completely eliminated for the total of 3 cases of women and 11 cases of men

Fig. 6: Assessment of itching symptoms at beginning of treatment (T0) and end of treatment (T2)8
THYMUSKIN® effectiveness in patients with androgenetic alopecia
- Hair loss reduced in an average of 96% of patients
- Seborrhoea and erythema improved in at least 89% of patients in each case
- Itching eliminated in 74% of women and 75% of men